Research Article - International Journal of Chemical and Pharmaceutical Analysis ( 2023) Volume 11, Issue 1
INVESTIGATION OF INHALED PHARMACOKINETICS OF RIBAVIRIN-LOADED SOLID LIPID NANOPARTICLES IN LUNG TISSUE OF ALBINO RATS
Namık Bilici, Ilknur Kulcanay Sahin, Omer F. Ersoy, Mustafa Cengiz*, Nurullah Ozdemir, Rıfat Ertekin and Adnan AyhanciMustafa Cengiz, Department of Mathematics and Science Education, Siirt University, Siirt, Turkey, Email: m.cengiz@siirt.edu.tr
Received: 28-Nov-2023, Manuscript No. IJCPA-23-121517; Editor assigned: 30-Nov-2023, Pre QC No. IJCPA-23-121517 (PQ); Reviewed: 14-Dec-2023, QC No. IJCPA-23-121517; Revised: 21-Dec-2023, Manuscript No. IJCPA-23-121517 (R); Published: 29-Dec-2023, DOI: 10.21276/2395-2466.23.11.087
Abstract
This study aims to investigate the inhaler pharmacokinetics of Ribavirin-Loaded Solid Lipid Nanoparticles (RSLN) in albino rats. After local ethical approval for animal experiments, rats were exposed to liquid RSLN with a particle size of 300 nm for 96 minutes in 7 groups (n=8), including six experimental and one control group. Then, serum and lung tissues were collected at ½,1,2,4,12, and 24 hours. HPLC-MS/MS analyzed the amount of Ribavirin (RBV) in serum and lung tissue after extraction. Serum Cmax 5.45 µg/mL, serum mean RBV concentration (CRBV) of groups 3.9512 (μg/mL), 3.1237 (μg/mL), 3.2181 (μg/mL), 2.9306 (μg/mL), 3.1268 (μg/mL), 3.16 (μg/mL) linear graph area of 3.16 (μg/mL) and 35, AUC-C/t 11 μl trap area of groups with 35, AUC-11µl linear graph was detected. Serum values were analyzed with One Way ANOVA (Analysis of Variance) statistics. The difference between groups was significant (p=0.40). Lung tissue RBV maximum concentration (PCRBV) Cmax was 3.78 µg/g, and the average lung tissue concentration of the groups was 2.544 (μg/g), 2.0085 (μg/g), 1.874 (μg/g), 1.2115 (μg/g), 0.73125 (μg/g), 0.4425 (μg/g), respectively. Lung tissue values were evaluated using the Kruskal-wallis way analysis method. According to this, the difference between groups was highly significant (p=0.01). The C/t graph area of the groups was found to be 15.66 µg/g-h by the same method. Data indicate that RBV provides adequate plasma concentration upon inhalation exposure. Evidence, that a particle size of 300 nm is sufficient to establish an effective serum concentration by exposure.
Keywords
Ribavirin, Antiviral inhaler drug, Ribavirin aerosol, Nano-pharmaceutical drug, Pharmacokinetics
Introduction
Inhaled anti-infective drugs play an essential role in preventing and treating respiratory tract infections. The complex aetiology and pathogenesis of respiratory viral infections seriously threaten human health. The most effective treatment is the administration of anti-infective agents directly to the respiratory tract. For this purpose, aerosols provide sufficient drug concentration to destroy pathogenic organisms [1]. Aerosolized administration greatly reduces the potential toxicity associated with systemic exposure. Despite the many advantages of aerosol delivery, research on the safety, efficacy, and pulmonary pharmacokinetics of anti-infective administered by this route still needs to be completed. Ways to optimize the benefit/risk ratio for inhaled drugs are possible with more PK/PD studies. Ribavirin (RBV) is not bound to plasma proteins and has a large volume of distribution (>2800 L) [2]. This may be due to the extensive transport of RBV into cells (including erythrocytes). Transport into non-plasma compartments may be via a stabilizing nucleoside transporter found in almost all cell types, which may explain RBV's large volume of distribution. Concentrations in erythrocytes continue to rise for approximately four days while plasma concentrations decrease. Following prolonged administration, the cerebrospinal fluid level may be up to 70% of the plasma concentration. Although it is uncertain whether the drug is transplacental in humans, there is accumulation and teratogenicity in erythrocytes in animal models. RBV is metabolized by phosphorylation to give a triazole carboxylic acid metabolite. RBV undergoes very little CYP-P450 enzyme-mediated metabolism. The elimination half-life is more than 40 hours [3]. The elimination half-life at 600 mg and 2 × 1 dose is 298 hours, possibly reflecting elimination from non-plasma compartments. The kidneys largely excrete RBV and its metabolites. The amount of RBV absorbed into respiratory secretions after nasal and inhalation vary depending on the method of administration, the concentration of the drug in the solution, and the length of administration time [4].
The pharmacokinetics of inhalable therapeutic agents differ from those given IV and enterally [1]. Compliance with the complexity of the physiological structure, lung health status, individual differences, molecule size, mechanics of administration, and pharmacokinetics of the molecule is of great importance. The high therapeutic effect, adequate concentration in the lung, and relatively low systemic absorption levels are aimed at inhaled administration. Therefore, in vivo, data is often simple, and it is necessary to use compromised, minimalist approaches that fit the ability to represent the physiological system [5]. For example; If the kinetic model assumes a single compartment with systemic absorption; If the rate constant of pulmonary mucociliary clearance, phagocytosis, or metabolism in parallel with the rate constant is called the rate constant of accumulation outside the lung, it is possible to find the plasma drug concentration curve against time following pulmonary administration in preclinical studies, and the ka and chan values for which bioavailability is calculated as follows [6].
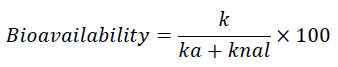
Ribavirin is a prodrug that is taken up by active transport into cells, where it is converted to its active metabolite RBV Triphosphate (RTP). It is one of the most studied molecules in inhaler aerosols. In bone marrow transplantation patients, lung transplantation patients, hematopoietic cell transplantation, RSV, cancerous Respiratory Syncytial Virus (RSV) patients, RNA viruses, Chronic Obstructive Pulmonary Diseases (COPD), acute bronchiolitis, influenza, in COVID-19, acute bronchiolitis, bronchiolitis [2,3,7-26]. There are many studies on RBV in viral treatment, pediatric viral infections, fatal influenza, respiratory papillomatosis, hepatitis-C, lower respiratory tract viral diseases, and protection from RSV [4,24,27-31]. Although there are not enough clinical studies, RBV Viral Haemorrhagic Fever (VHF), Colorado tick fever virus, Crimean-Congo hemorrhagic fever virus, Cytomegalovirus (CMV), Hantaan virus, hepatitis A virus, herpes simplex virus types I and II, Human Immunodeficiency Virus (HIV), influenza A and B virus, Junin virus, Lassa virus, measles virus, Cannabis virus, parainfluenza virus, many viral infections such as inovirus, Rift Valley fever virus, rotavirus, subacute sclerosing panencephalitis virus, variola virus (smallpox), West Nile virus, yellow fever virus have been studied and most of them have been successful [32-34]. Data indicate that RBV provides adequate plasma concentration upon inhalation exposure. This study aims to investigate the inhaler pharmacokinetics of Ribavirin-loaded Solid Lipid Nanoparticles (RSLN) in albino rats.
Materials and Methods
The water-soluble powder form of ribavirin (99.5%) was obtained from Jinan Mingxin Pharmaceutical Co., ltd, and compritol and polyoxyethylene sorbitan monooleate (Tween 80) were obtained from Merck® Schuchardt (Darmstadt, Germany). Heparin tube, Phosphate Buffered Saline pH=7.4 (PBS), nebulizer, and other consumables for the experimental animals were obtained from Turkey.
Solid lipid nanoparticle preparation of ribavirin
The hot homogenization technique described by Müller was used in the preparation of Ribavirin Solid Lipid Nanoparticle (RSLN) [35]. A lipid solution was prepared at 80ºC with heat using 5% RBV, 5% lipid fluid (Compritol 888), and 3% surfactant (Tween 80). RBV was added to the lipid solution, and Tween 80 was slowly and slowly mixed with Ultra-Turrax (T25, Janke and Kunkel IKA®, Germany) for 10 minutes. Then, the RSLN complex was obtained by mixing at 20,500 rpm for 1 minute. The solution containing the nanoparticle system obtained by passing through a complex 0.2μm filter was cooled to room temperature. It was stored in sterile and colored glass tubes with screw caps. SLNs were repeated for control by applying the same procedures without using RBV.
Characterization of RBV-loaded Solid Lipid Nanoparticles (RSLN)
The mean diameters (particle size) and Polydispersity Index (PI) of SLNs were determined by Photon Correlation Spectroscopy (PCS) using a Nano Zetasizer (ZS, Malvern, UK) at a fixed angle of 90° and temperature of 25°C. The zeta potential was measured at 25°C via a Nano Zetasizer to assess the colloidal distribution's stability. SLNs were then suspended in distilled water (pH=7). Each of the samples was analyzed in triplicate. SLNs were spread on a Cu grid, stained with uranyl acetate, and examined under Transmission Electron Microscopy (TEM). Ribavirin-loaded SLNs were validated with TEM (TEM FEI Tecnai™ Bio Twin). The zeta potential of the particles was determined using Malvern Zetasizer Nano ZS (Malvern Instruments). RSLN particle size averaged 4188 nm (± 3.26), and PI averaged 0.376 ± 0.05 (n=3).
Application method to experimental animals
The European Union Directive on the protection of animals used for scientific purposes numbered 2010/63/EC (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010) [36]. The regulation numbered 28141, dated 13.12.2011, which adopted the provisions of the law as a basis, was complied. First, permission was obtained from the local ethics committee of Zonguldak Bülent Ecevit University Experimental Research Application and Research Center (DEHAM) Animal experiments with the number 2021/05 on 03.06.2021.
The prepared RSLN drug was given by passive inhalation in aerosol form without limiting the animals' awake and normal respiration. In the indoor environment, the average temperature was 22.5°C, and the humidity was kept at 55%-65%. A nebulizer was used to deliver aerosol drugs. Whole-body exposure was preferred for delivering liquid or solid drugs to the lungs since it is a non-invasive, problem-free method [7,10,31,37].
In the study, eight weeks old Wistar albino 350g (± 20gr) male rats were studied. Throughout the study, rats were fed ad-libitum with commercial pellets that met all dietary requirements. They were allowed to drink water (ad-libitum). The rats were kept at an ambient temperature of 22°C (± 2) and in a well-ventilated (55%-60% humidity) environment. They were divided into seven groups in separate cages after a 5-day adaptation period in a 12-hour light and 12-hour dark cycle. In order to relieve experimental stress in all groups, a 90-minute exercise period was applied twice in the morning and evening for two days. The sham group was used instead of the control group. It was planned to have 8+2=10 rats in each group. Eight experiments were kept, two of which were used for possible loss compensation. There were no losses. Therefore, 2 animals were not used.
The environment of the experimental groups (6 groups) was kept the same. Nebulized budesonide at a dose of 0.5 mg/mL for 10 minutes was administered to all groups (n=7) before the experiment, with an airflow of 10 L/min. The experiment for all groups was carried out in a 0.6 m3 polypropylene transparent container. In the upper 1/10 of the test vessel, an opening of 1 cm in diameter was left on both sides, one is suitable for mutual air inlets and outlets, and one is free from the nebulizer hose inlet. Humidity and temperature were measured instantaneously. ACE-certified nebulizer with a compressor was used for the experiment. The airflow compressed by the nebulizer per unit time was adjusted to 10 L/min and 0.5 mL/min drug consumption. The drug dose was used by calculating 20 mg/kg over 20 mg/mL solution [19,38]. When the passive inhaler dose was 20 mg/mL, the dose was predicted to be 7 mg/live weight for 350 g rat [19,21]. The drug dose was calculated based on 7.2 L/h passive breathing over the mean tidal volume. Our RSLN solution was at a concentration of 6,25 mg/mL. For the rat dose of 7 mg/rat, the drug exposure time was kept at 96 minutes. The drug dose was calculated over D=C × F × Vt × T. Lung accumulation was also taken as C × T × RMV × Df (Df=Deposition factor=1/10 for rat). (51.54). 8 minutes of prodrug aerosolization was performed for sufficient drug concentration of the medium. RSLN was given to 6 groups and 0.9% NaCl (isotonic) to the control group. Then, at the end of ½,1,2,4,12, and 24 hours, Xylazine (10 mg/kg) X ketamine (75 mg/kg) was euthanized by deep anesthesia. >2 mL blood from A. abdominals was taken into heparinized tubes. Lungs were separated from the surrounding tissues and placed in 50mL tubes containing 20 mL PBS (pH=7.4). Blood samples and tissue samples were numbered together and kept at -40°C. The samples were delivered to Tekirdağ Namık Kemal University Instrumental Analysis Laboratory (NABİLTEM) in cold storage. Samples were prepared for analysis with the specified method [3]. Blood samples were vortexed for 1 minute. It was centrifuged at 4500 rpm for 10 min. 100 µL of serum obtained in the upper phase was taken. 900 µL of Acetonitrile (ACN) was added to it. It was vortexed for 15 seconds. It was centrifuged at 13,000 rpm for 10 minutes at room temperature. It was diluted with acetonitrile, filtered through a 0.22 µm syringe filter, taken into a vial, and made ready for injection into the device. 1g of lung samples was taken, and 10 mL of ACN was added and homogenized with a homogenizer. After vortexing for 2 minutes, it was centrifuged at 4500 rpm for 10 minutes. It was filtered through a 0.22 µm syringe filter, diluted with acetonitrile, and made ready for injection into the device.
Analyzes were performed on an AB Sciex brand and 3200 QTRAP (AB Sciex, Foster City, USA) model High-Pressure Liquid Chromatography Mass Spectrometry (HPLC-MS/MS) instrument. Agilent Proshell 120 SB: C18 2.7 µm 100 × 3.0 mm column was used. Acetonitrile (20%) and deionized water (0.1% Formic acid) were used as the mobile phase. The flow rate was set to be 0.2 mL/min and the injection volume to be 10 µL. The method of application to the device, analytical device parameters, and MRM data are given in Table 1.
MS/MS Terms; AB Sciex 3200 QTRAP was used as a mass spectrophotometry detector. Ionization was performed in positive or negative ion mode using the Electrospray Ionization (ESI) module. The scanning type was set as MRM (Multiple Reaction Monitoring), capillary voltage 4500V, curtain gas (30 psi), collision gas (medium), and ion source gas (50 psi) using nitrogen gas. The temperature of the TurboIon Spray module was fixed at 500℃. Analyte dependent parameters; Working standard solution containing 0.1 mg/kg of standard substance was used for DP (Declustering Potential), CE (Collision Energy), and CXP (Cell Exit Potential). Data analysis and graphics were obtained from the special program of the device based on Excel.
Results
In this study, which we conducted with the 300 nm RSLN we prepared previously, no respiratory distress or death occurred in any of the experimental animals. All of them were monitored, including those kept for up to 24 hours. No changes were observed in respiration, food intake, and water drinking. In the first group (1/2h) of rats administered RSLN by aerosolization, the group's mean was calculated starting from the highest serum concentration of 5.45 µg/mL to the lowest level of 2.73 µg/mL. The mean of the first group was determined as 3.95 µg/mL. For the 2nd group (1h), the highest serum RBV concentration was 3.99 µg/mL, the lowest was 2.51 µg/mL, and the group mean was 3.12 µg/mL, respectively. At the end of the 2nd hour, the highest serum concentration was 3.88 µg/mL, the lowest was 2.35 µg/mL, and the group mean was 3.22 µg/mL. At the end of the 4th hour, the blood concentration was 3.5 µg/mL, the lowest was 1.68 µg/mL, and the group mean was 2.93 µg/mL. After 12 hours, the highest blood concentration was 4.01 µg/mL, the lowest was 2.43 µg/mL, and the group mean was 3.13 µg/mL. After 24 hours, the highest blood concentration was 3.81 µg/mL, the lowest was 2.29 µg/mL, and the group mean was 3.16 µg/mL. No drug residues were found in the control group. The mean serum RBV concentrations of the groups at 1/2,1,2,4,12, and 24 hours were sorted from largest to smallest in µg/mL and plotted (Figure 1). The mean serum RBV concentrations of the groups (excluding the 4th hour) were between 3.95 (µg/mL/30 min) and 3.16 (µg/mL/24 hours). It was 2.93 µg/mL in 4 hours. The mean serum RBV concentration of the groups over time was given (Figure 2). The area under the curve AUC (Area Under the Curve) was calculated with the linear trapezoidal method and given in Figure 3. A high blood concentration indicates that respiration rapidly absorbs ribavirin into the blood. It reveals that ribavirin is metabolized slowly while it is attached to blood cells on the one hand. While the lung concentration of ribavirin given a single dose decreased rapidly, the blood concentration tended to decrease after 4 hours.
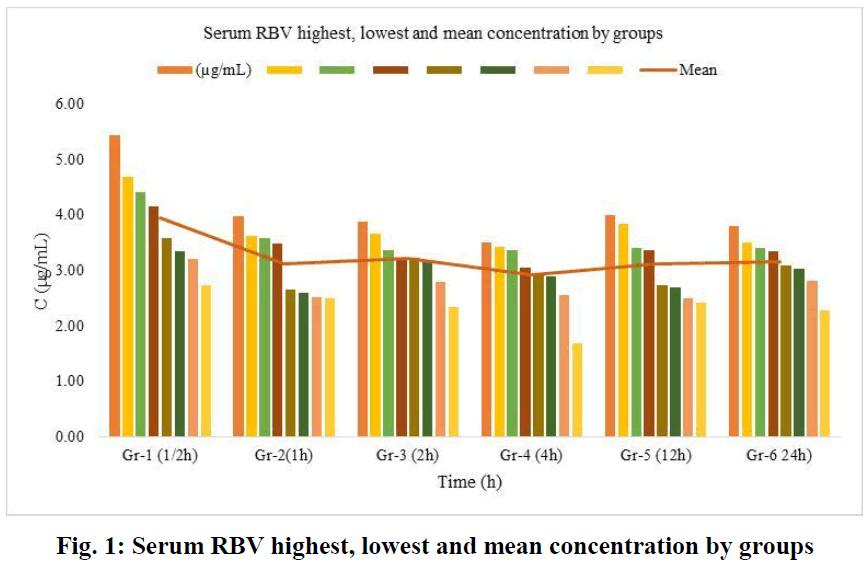
Fig. 1: Serum RBV highest, lowest and mean concentration by groups
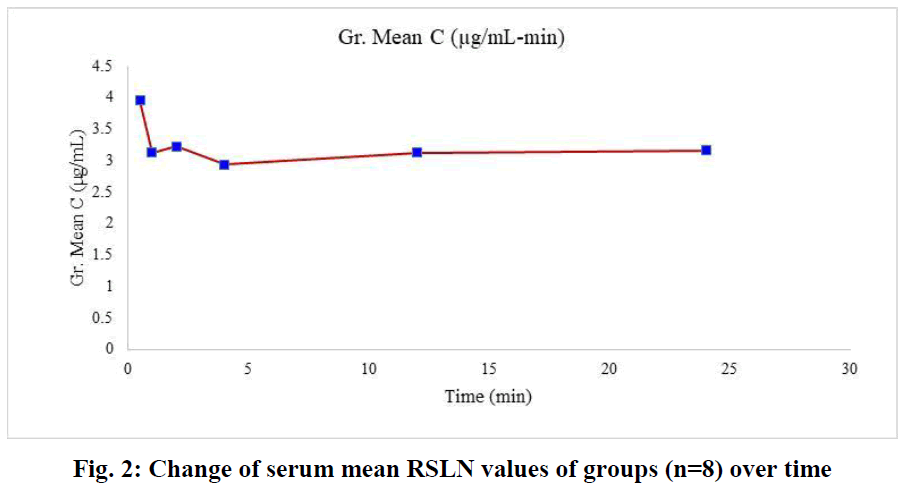
Fig. 2: Change of serum mean RSLN values of groups (n=8) over time
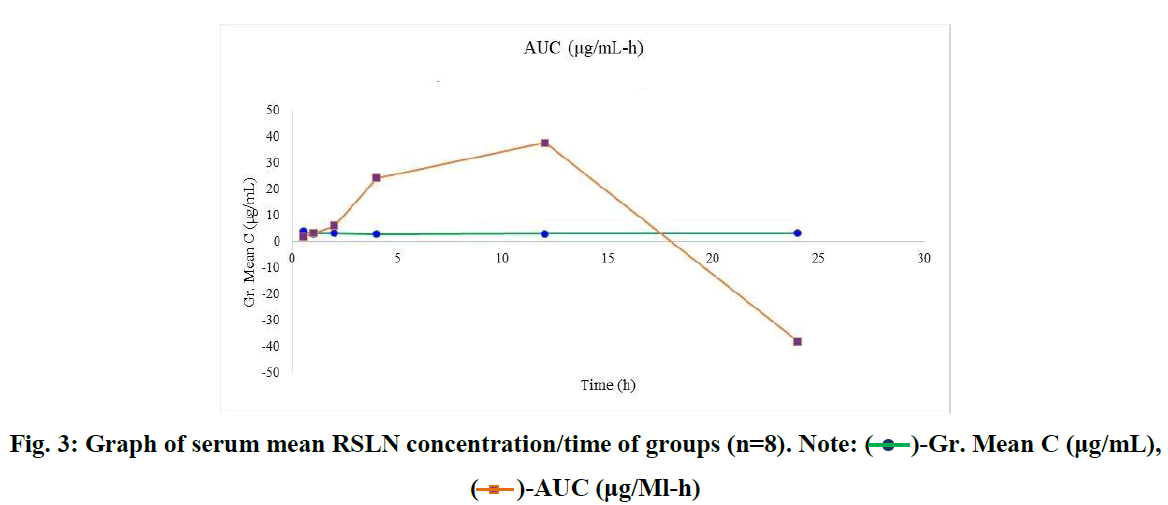
Fig. 3: Graph of serum mean RSLN concentration/time of groups (n=8). Note:  -Gr. Mean C (μg/mL),
-Gr. Mean C (μg/mL),  -AUC (μg/Ml-h)
-AUC (μg/Ml-h)
For RBV lung tissue concentrations, within-group tissue concentrations of 6 groups (n=8) were listed (Figure 4). The course of the group averages was followed from the first group (1/2 h) to the last group after 24 h. RBV concentrations accumulated in the lung tissue were ordered from the highest (2.544 µg/g) to the lowest (0.442 µg/g) and averaged (1.47 µg/g) (Figure 5).
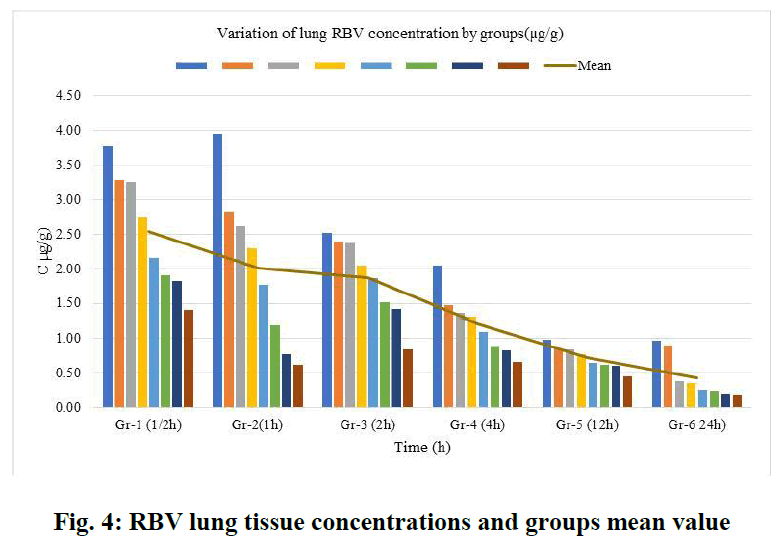
Fig. 4: RBV lung tissue concentrations and groups mean value
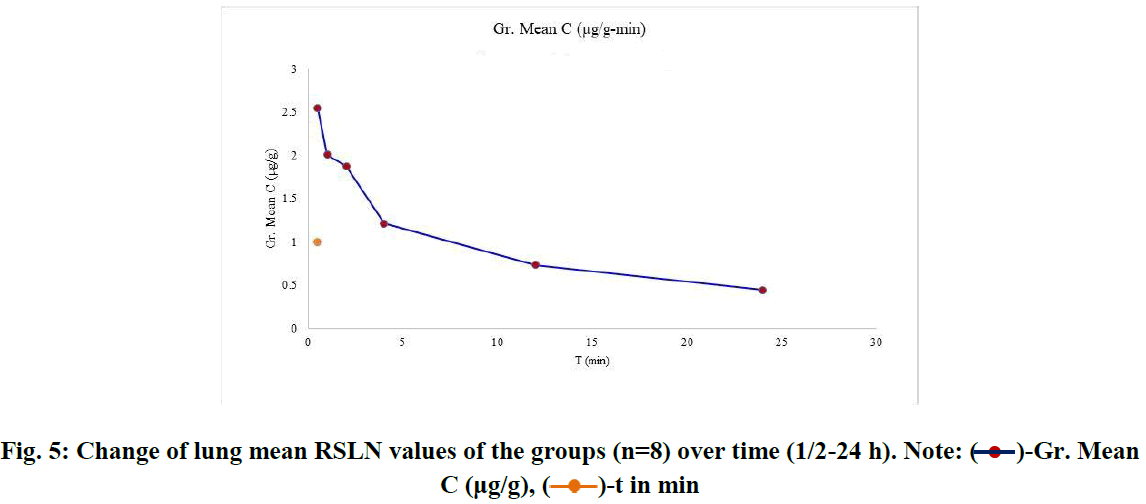
Fig. 5: Change of lung mean RSLN values of the groups (n=8) over time (1/2-24 h). Note:  -Gr. Mean C (μg/g),
-Gr. Mean C (μg/g),  -t in min
-t in min
RSLN lung tissue concentration was highest at 3.78 µg/g, lowest at 1.4 µg/g, and group mean at 2.544 µg/g in 1/2 hour. In the 1st hour, the highest was 3.96 µg/g, the lowest was 0.628 µg/g, and the group mean value was 2.0085 µg/g. At the end of the 2nd hour, the highest was 2.52 µg/g, the lowest was 0.842 µg/g, and the group mean value was 1.874 µg/g. At the end of the 4th hour, the highest was 1.488 µg/g, the lowest was 0.672 µg/g, and the group mean value was 1.2115 µg/g. At the 12th hour, the highest 0.982 µg/g, the lowest 0.466 µg/g, and the group mean value were found to be 0.7312 µg/g. At the 24th hour, the highest 0.978 µg/g and the lowest 0.1908 µg/g and group mean value were determined as 0.4425 µg/g. The area under the curve was calculated using the linear trapezoidal method and given graphically (Figure 6). The result found by the Linear Trapezoidal Method (AUC=1/2(C1+C2)(t2-t1) was divided by the total elapsed time (0,652 µg/g-24h). Statistical data are given in Table 2.
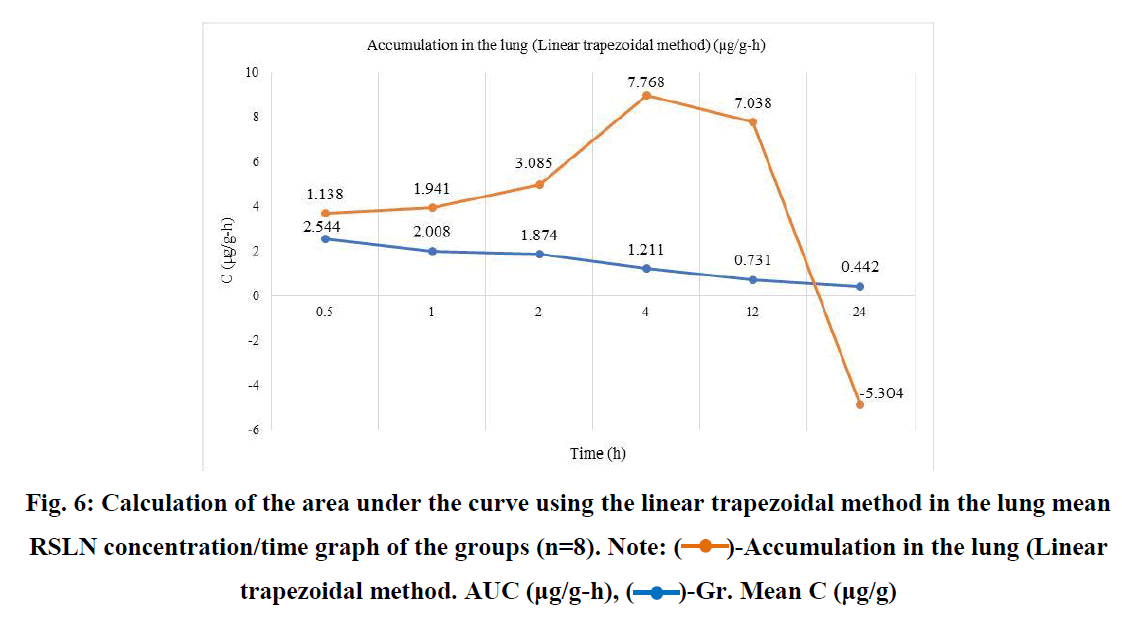
Fig. 6: Calculation of the area under the curve using the linear trapezoidal method in the lung mean RSLN concentration/time graph of the groups (n=8). Note:  -Accumulation in the lung (Linear trapezoidal method. AUC (μg/g-h),
-Accumulation in the lung (Linear trapezoidal method. AUC (μg/g-h),  -Gr. Mean C (μg/g)
-Gr. Mean C (μg/g)
RSLN lung tissue concentration analysis 50 ppm spike, lung tissue RBV calibration, and RBV ion were monitored in HPLC-MS/MS device (Figures 7-9).
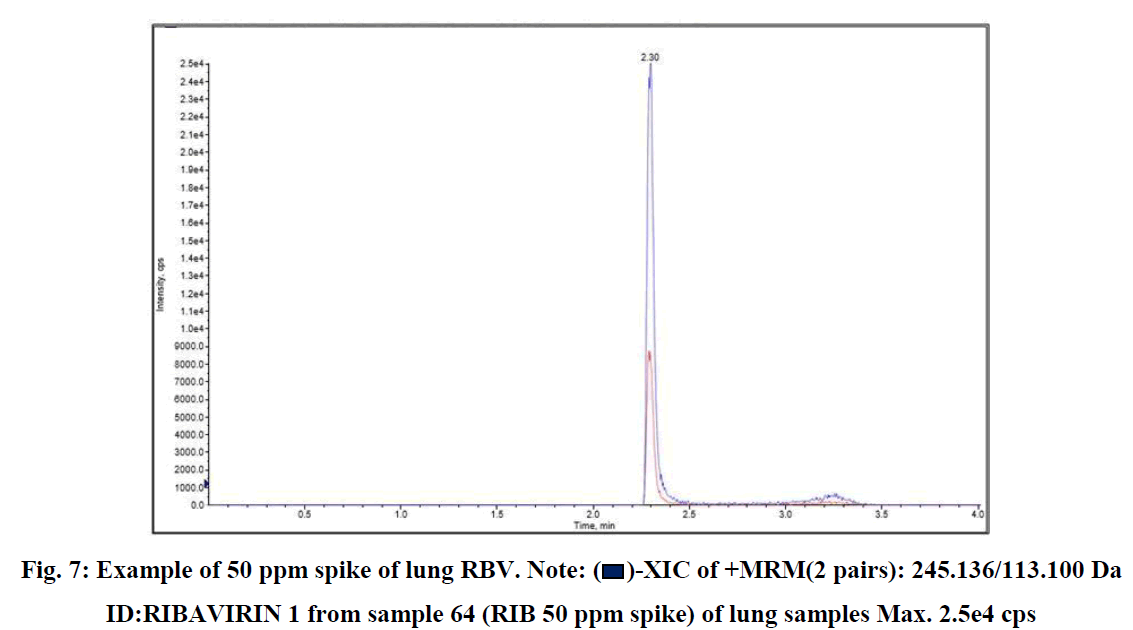
Fig. 7: Example of 50 ppm spike of lung RBV. Note:  -XIC of +MRM(2 pairs): 245.136/113.100 Da ID:RIBAVIRIN 1 from sample 64 (RIB 50 ppm spike) of lung samples Max. 2.5e4 cps
-XIC of +MRM(2 pairs): 245.136/113.100 Da ID:RIBAVIRIN 1 from sample 64 (RIB 50 ppm spike) of lung samples Max. 2.5e4 cps
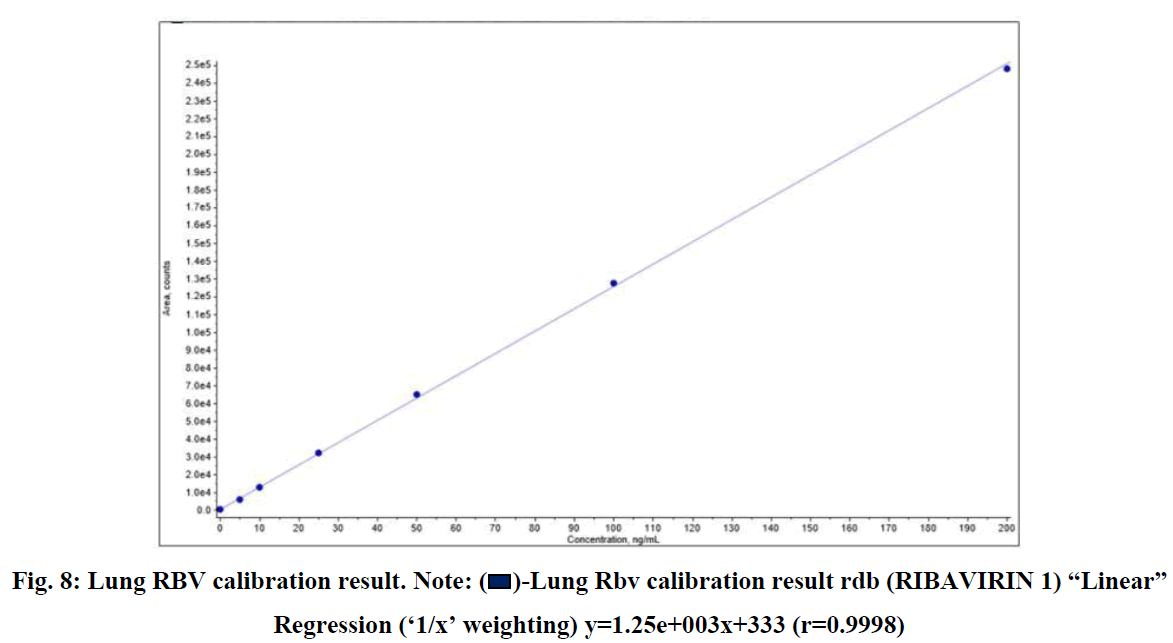
Fig. 8: Lung RBV calibration result. Note:  -Lung Rbv calibration result rdb (RIBAVIRIN 1) “Linear” Regression (‘1/x’ weighting) y=1.25e+003x+333 (r=0.9998)
-Lung Rbv calibration result rdb (RIBAVIRIN 1) “Linear” Regression (‘1/x’ weighting) y=1.25e+003x+333 (r=0.9998)
Fig. 9: Ribavirin product ion in lung sample. Note:  -+MS2 (245.14) CE(50): 26 MCA scans from sample 1 (Tune sample name) of RIBAVIRIN_InitProduct_Pos.wiff (Turbo spray)
-+MS2 (245.14) CE(50): 26 MCA scans from sample 1 (Tune sample name) of RIBAVIRIN_InitProduct_Pos.wiff (Turbo spray)
Discussion
As a route of administration for end-organ or systemic therapies in treating pulmonary diseases, the lung is a very attractive target for drug release. The lung is the only organ through which the entire cardiac output passes. It is the best-perfused organ in the body as it receives the entire cardiac output. So it may provide an enormous surface area for the systemic absorption of drugs. Its low enzymatic interaction also provides a controlled environment. It therefore, allows direct access to the disease in treating respiratory diseases. The lung exists to prevent unwanted particles in the air from entering the body under normal conditions. Airway geometry, moisture, mucociliary clearance, and alveolar macrophages play a vital role in maintaining the sterility of the lung. However, these are also barriers to the therapeutic efficacy of inhaled drugs [38]. Several hurdles must be overcome before an inhaled drug can be absorbed from the lung into the blood: lung surfactant, surface coating fluid, epithelium, interstitium, basement membrane, and endothelium. A thin alveolar-vascular permeable barrier regulates drug absorption. The number of alveoli ranges from 200 to 600 million, creating an enormous epithelial surface area comprising a single thin cellular layer (0.2-0.7 µm thick). The high bioavailability of macromolecules deposited in the lung (10-200 times greater than nasal and gastrointestinal values) is owed to this enormous surface area. Ultimately, direct delivery of drugs to the lungs; provides a rapid clinical response, reduced risk of undesirable side effects, escape from first-pass metabolism, independence from gastrointestinal system problems, the possibility of treatment with a very small dose, being free from many enzymatic inactivations, absorption with an average of 100 m2 of enormous permeable absorbent surface in the alveolar region, ease of excretion and a non-invasive application advantage.
Treatment efficiency varies according to the methods of using inhaled drugs in lung diseases. One of the important factors affecting treatment efficiency is the structure of the drug molecule. Molecules that are easy to dissolve in water, such as ribavirin, are important and advantageous [39,40]. Today, sophisticated techniques and different uses of drugs are available. Optimization of the physicochemical properties of different molecules with various techniques pushes the genius of engineering [21,41]. It includes the various molecular structures of RBV. With the use of RBV in aerosol form by dissolving it in water in the past, studies conducted by many researchers [42,43], have been adopted by health authorities, and its use in treatment has been increased. The ability of RBV to be transported to the lungs in the form of an aerosol that can be administered to the depths of the lung tissue and the systemic circulation is possible if the molecular characterization and its carriers are compatible and efficient. According to the common opinion, the most suitable particle size is 3 µm RBV molecules. It is considered efficient that the drug molecule is 1-5 µm in size, and it is known that 3µ particles are used most effectively in lung therapy. Apart from RBV, anti-inflammatory steroids, β2 agonists for asthma, and antibacterial and antimycotic drugs have also been shown to penetrate the lungs more deeply when aerosols of different sizes (1.5 µm, 2.8 µm, and 5 µm) are given in equal doses. These particles were found to be more effective in small airways. Only a local effect was obtained from the larger particles filtered in the upper respiratory tract [44-46]. The long terminal half-life of ribavirin (120-170 h) makes both pulmonary and systemic bioavailability ideal when administered as an aerosol. Therefore, with a drug with a well-designed molecular design, the entire lung surface area becomes the target treatment area. It allows a high accumulation of drug molecules that cannot be compared with enteral administration routes [39,47].
The difficulty with therapeutic aerosols is their ability to transcend the lung's various lines of defense. Drug particles deposited in the conducting airways are primarily removed by mucociliary clearance and, to a lesser extent, absorbed into the blood or lymphatic system via the airway epithelium. In addition, the rheology of the mucus must be within the physiological range. Most particles larger than 10 µm attach to the larynx or accumulate in the oropharyngeal region. It is then swallowed and contributes minimal or no therapeutic response. Since the air velocity is negligible in the alveolar region, precipitation by inertia impact is zero. Deposition due to sedimentation affects particles up to 0.5 µm in diameter, while below 0.5 µm, the main sedimentation mechanism is diffusion. The actual deposition site is difficult to predict because airway caliber and anatomy differ between humans. However, overall, aerosols 5-10 µm are destined mainly in the large conductive airways and oropharyngeal region, particles 1-5 µm in diameter in small airways, >50% of particles ≤ 3 µm in diameter are directed to the alveolar [48]. In this study, the ideal particle size for the lung (3µ) was tested in producing and characterizing ideal RSLN molecules [35]. Excluding drug wastage and price through whole-body exposure, its efficacy is kinetic [49]. When the drug is given by free inhalation, its adaptation to humans can be calculated by proportioning the surface area [15,27,34,37,50]. Preclinical data on clinical inhaled drugs are adapted using a fixed allometric exponent of 0.67. These estimated allometric calculations for RBV, depending on age and other variables, have been made for many years [1,19,34].
The delivery method is as important as the appropriate particle size (mass median diameter) in efficiently delivering the molecule to the lung. Similar to human models, experimental animals take drugs by free breathing in a closed environment without behavioral restrictions. All aerosol drugs whose target is humans can be used by adapting studies on experimental animals to humans. Although some methods that limit force or invasive respiration are more efficient, similar use in humans is impossible. The situation is similar in experiments performed under anesthesia. Regardless of the method of administration, the delivery of drugs to the lungs and distribution to the central compartments has different PK/PD data from the normal routes of use [38,51,52]. In aerosol therapies, the accumulated dose and distribution in the lung limit the therapeutic effect. The receptor site or permeable membrane region in the lung limits the efficacy of drug aerosol [53]. For example, the location of the receptors in the lung suggests that ipratropium bromide should be delivered to conductive airways.
In contrast, salbutamol should be delivered more peripherally to the medium and small airways to produce a therapeutic effect. Unlike bronchodilators, inhaled anti-inflammatory therapy is most beneficial when distributed evenly throughout the lung, as inflammatory cells are found in the airways and alveolar tissue. Therefore, aerosol particle size properties may play an important role in efficacy in targeting the drug to the appropriate lung region and avoiding physiological barriers of the lung [41].
RSLN shows rapid accumulation in the lungs. Even with aerosolization, a certain blood concentration can be achieved in a very short time. Ribavirin concentrations occur two to three times higher even after high-dose, short-term treatment [43]. İt causes the accumulation of enterally and parenterally administered drugs in target organs outside the lung in lower amounts than those administered by aerosolization [51]. In studies, the accumulation in the brain is interpreted as the first-pass effect of drugs given other than aerosolization [52]. Although the dose given by aerosolization in the form of exposure is wasted, direct delivery to the lung as a target, very wide lung absorption surface, optimum particle size (3µ), and being away from anesthesia and stress of the subjects increase the level of presence in the central compartment [7,37,46,53].
The efficacy of ribavirin, including passive inhalation, has been experimentally tested in mice, cotton rats, ferrets, rats, guinea pigs, monkeys, and dogs [3,43,54]. Nebulizer is widely used effectively and efficiently today in delivering drugs to the lungs in humans. Although drug delivery with a nebulizer is easier than intra-tracheal instillation of large volumes of fluids, losses in reservoir drug dose and dosing difficulties are important dilemmas. Significant loss of drug dose occurs in the reservoir of the aerosol generator, piping, animal, accessories (aerosolization chamber), and during exhalation of the animal. It has been argued that passive inhalation cannot be preferred for expensive drugs because this loss causes the dose not to be well controlled [21,49]. However, since other methods have difficulties or require invasive intervention, their use in humans is limited. Despite the waste of the drug, the applicability of animal experiments with passive inhalation away from stress to humans seems more reasonable. As a matter of fact, systems that allow passive inhalation are used in infants and children by closing the head-neck region [10,53]. In the case of total exposure, the dose calculation can be formulated as follows.
The total inhaled dose is calculated over C (mg/L) × T(min) x RMV (L/min) [3,45]. Although there are losses in this formulation, the bioavailability can be calculated approximately based on the respiratory volume per minute and the dose. The dose losses of drugs that undergo first-pass effect, are not resistant to stomach acid, or are highly affected by intestinal fermentation are not small. In addition to these large losses of entirely administered drugs, their presence in the respiratory tract is lower than those given by aerosolization. On the other hand, in enteral drug use, the active molecule is loaded on the whole organism. In drugs administered by passive aerosolization, the target is the lungs. Since transmission occurs directly, there is an effective regional treatment [41,52,55].
Although the blood RBV concentration is relatively higher in the first two hours, a portion of the virus has sufficient MIC for 24 hours. Studies measured serum RBV concentration as 3.95 µg/mL 30 minutes after a single dose aerosol application. MIC is sufficient for most known DNA and RNA viruses [52,56]. The fact that the blood concentration is 3.25 µg/mL for 24 hours with a single inhalation dose can be explained by the molecular character of RBV, its retention in blood cells, and its intracellular penetration ability [10]. It would not be wrong to say it like this. RBV tends to collapse over time as it is rapidly converted to its phosphorylated derivatives. Ribavirin Triphosphate (RTP) is similar in structure to ATP. For this, it competitively inhibits ATP- dependent energy use in red blood cells. Due to the absence of dephosphorylation enzymes in erythrocytes, RTP accumulates over time [14]. İt can also lead to hemolytic anemia. The severity of anemia is highest in cynomolgus monkeys, followed by humans, rats, and dogs [32].
The RSLN molecule we characterize can provide maximum plasma concentration in half an hour with a 96 hour inhalation. The blood concentration obtained with inhaled RSLN was similar to previous oral ribavirin PK studies. However, it is lower than the concentration created by the IV dose. The tendency for total body exposure to decrease over 24 hours is quite slow. This may be due to continued oral ingestion of RBV residues. After a single dose of RBV whole-body exposure, the concentration calculated by the linear trapezoidal method for 24 hours was 35.11 µg/mL. Although higher than oral use, a lower concentration than IV administration can be mentioned. This level of concentration is sufficient for most RNA-carrying viruses [3]. In the case of drug administration in the form of nanoparticle aerosol exposure, the efficiency of the whole body exposure, the freedom to be used without stress, the freedom to be used unlimitedly, the simplicity and cheapness of the mechanism, and the ability to control the temperature, humidity, and gas flow with the drug are the superior aspects [37,46]. Lung tissue RBV level is detectable even after 24 hours with a gradual, steady decrease (2.54-0.44 µg/g). Although the lung tissue RBV concentration decreases at 1/2-24 hour level, its average is 1.47 µg/g. Although this decreasing trend falls below 1 µg/g after the 4th hour, it is possible to detect RBV at 0.4 µg/g in the lung tissue even after 24 hours [36]. Similarly, in comparing the ribavirin inhaler standard and short-term/high-dose treatment in RSV patients, short- term high-dose treatment was effective [14]. Indeed, researchers found a survival rate of over 90% after a single 30-minute exposure on days 1-4 in treating influenza A and B infection. Therefore, there is success in short-term treatments without resistance [38].
Conclusion
Research in the field of pulmonary drug delivery has gained momentum in recent years. With increasing interest in using the lungs as a delivery vehicle systemically, technological advances have led to the development of more efficient delivery systems that deliver larger doses and finer particles to the lung. As more efficient pulmonary delivery devices and sophisticated formulations become available, physicians and healthcare professionals will choose various device and formulation combinations to target specific cells or regions of the lung, avoiding and retaining the lung's clearance mechanisms. Just having inhalation therapy is not enough for prescribing. Therefore, it is necessary to focus on treatments that are easily applicable and prioritize patient welfare, both in the drug's molecular character and the efficiency of the treatment. Although the differences between the groups in the blood concentration of the given RSLN drug are remarkable, accumulation in the lung is highly significant (p<0.001). A sufficient and significant concentration occurs from the 4th hour after ingestion of the drug through the lung. Although dose calculation of whole-body exposure, drug cost, and PK/PD efficiency level is problematic, It is applicable to avoid stress and to take environmental conditions under control. Apart from the invasive methods used in treatment, all body openings are worth evaluating by developing sophisticated new methods as potential treatment targets. This situation; will ensure the continuous development of new ideas, practices, and efficiency regarding the future of treatment methods. Just as the conventional treatment potential for diabetes, contraception, and chemotherapeutic areas has been optimized in the last 80 years, aerosol treatment methods can be optimized over time by minimizing their errors.
Acknowledgement
This experimental research was supported by the Scientific Research Projects Unit of Karabuk University with the no. KBÜBAP-22- KP-152. I am grateful for this support.
Conflict of Interest Statement
The authors of this publication have no conflicts of interest among themselves or with the institutions.
References
- Knight V, Yu C, Gilbert BE, Divine GW. Estimating the dosage of ribavirin aerosol according to age and other variables. J Infect Dis. 1988;158:443-448.
[Crossref] [Google Scholar] [PubMed]
- Li L, Avery R, Budev M, Mossad S, Danziger-Isakov L. Oral versus inhaled ribavirin therapy for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant. 2012;31:839-844.
[Crossref] [Google Scholar] [PubMed]
- Lin CC, Yeh LT, Luu T, Lourenco D, Lau JY. Pharmacokinetics and metabolism of [14c] ribavirin in rats and cynomolgus monkeys. J Antimicrob Agents. 2003;47:1395-1398.
- Imwong M, Pukrittayakamee S, Looareesuwan S, Pasvol G, Poirriez J, White N, et al. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: Geographical and clinical correlates. J Antimicrob Agents. 2001;45:3122-3127.
[Crossref] [Google Scholar] [PubMed]
- Rodriguez WJ, Hall CB, Welliver R, Simoes EA, Ryan ME, Stutman H, et al. Efficacy and safety of aerosolized ribavirin in young children hospitalized with influenza: A double-blind, multicenter, placebo-controlled trial. J Pediatr. 1994; 125:129-135.
[Crossref] [Google Scholar] [PubMed]
- Ferrati S, Wu T, Fuentes O, Brunaugh AD, Kanapuram SR, Smyth HD. Influence of formulation factors on the aerosol performance and stability of lysozyme powders: A systematic approach. AAPS PharmSciTech. 2018;19:2755-2766.
[Crossref] [Google Scholar] [PubMed]
- Arnold SD, Buchan RM. Exposure to ribavirin aerosol. Appl Occup Environ Hyg. 1991;6:271-279.
- Seo S, Campbell AP, Xie H, Chien JW, Leisenring WM, Englund JA, et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: Significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013;19:589-596.
[Crossref] [Google Scholar] [PubMed]
- Shah DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68:1872-1880.
[Crossref] [Google Scholar] [PubMed]
- Caramia G, Palazzini E. Efficacy of ribavirin aerosol treatment for respiratory syncytial virus bronchiolitis in infants. J Int Med Res. 1987;15:227-233.
[Crossref] [Google Scholar] [PubMed]
- Conrad DA, Christenson JC, Waner JL, Marks MI. Aerosolized ribavirin treatment of respiratory syncytial virus infection in infants hospitalized during an epidemic. J Pediatr Infect Dis. 1987; 6:152-158.
[Crossref] [Google Scholar] [PubMed]
- De Clercq E. Chemotherapy of respiratory syncytial virus infections: The final breakthrough. Int J Antimicrob Agents. 2015;45:234-237.
[Crossref] [Google Scholar] [PubMed]
- Edell D, Bruce E, Hale K, Edell D, Khoshoo V. Reduced long‐term respiratory morbidity after treatment of respiratory syncytial virus bronchiolitis with ribavirin in previously healthy infants: A preliminary report. Pediatr Pulmonol. 1998;25:154-158.
[Crossref] [Google Scholar] [PubMed]
- Englund JA, Piedra PA, Jefferson LS, Wilson SZ, Taber LH, Gilbert BE et al. High-dose, short-duration ribavirin aerosol therapy in children with suspected respiratory syncytial virus infection. J Pediatr. 1990; 117:313-320.
[Crossref] [Google Scholar] [PubMed]
- Hall CB, McBride JT, Walsh EE, Bell DM, Gala CL, Hildreth S et al. Hall, Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection: A randomized double-blind study. J Med. 1983;308:1443-1447.
[Crossref] [Google Scholar] [PubMed]
- Jefferson LS, Coss‐Bu JA, Englund JA, Walding D, Stein F. Respiratory system mechanics in patients receiving aerosolized ribavirin during mechanical ventilation for suspected respiratory syncytial viral infection. Pediatr Pulmonol. 1999;28:117-124.
[Crossref] [Google Scholar] [PubMed]
- Caufield JH, Zhou Y, Garlid AO, Setty SP, Liem DA, Cao Q, et al. A reference set of curated biomedical data and metadata from clinical case reports. Sci Data. 2018;5:1-18.
[Crossref] [Google Scholar] [PubMed]
- Permpalung N, Thaniyavarn T, Saullo JL, Arif S, Miller RA, Reynolds JM et al. Oral and inhaled ribavirin treatment for respiratory syncytial virus infection in lung transplant recipients. Transplantation. 2020;104 :1280-1286.
[Crossref] [Google Scholar] [PubMed]
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Clin Pharm. 2016;7:27.
[Crossref] [Google Scholar] [PubMed]
- Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: Direct molecular test by using ribavirin. Proceedings of the National Academy of Sciences. 2001;98:6895-6900.
[Crossref] [Google Scholar] [PubMed]
- Dumont EF, Oliver AJ, Ioannou C, Billiard J, Dennison J, Van den Berg F, et al. A novel inhaled dry-powder formulation of ribavirin allows for efficient lung delivery in healthy participants and those with chronic obstructive pulmonary disease in a phase 1 study. J Antimicrob Agents Chemother. 2020;64:02267-02219.
[Crossref] [Google Scholar] [PubMed]
- Newman SP. Delivering drugs to the lungs: The history of repurposing in the treatment of respiratory diseases. Adv Drug Deliv Rev. 2018;133:5-18.
[Crossref] [Google Scholar] [PubMed]
- McClung HW, Knight V, Gilbert BE, Wilson SZ, Quarles JM, et al. Ribavirin aerosol treatment of influenza B virus infection. JAMA. 1983;249:2671-2674.
[Crossref] [Google Scholar] [PubMed]
- Knight V, Gilbert BE. Aerosol treatment of respiratory viral disease. Lung. 1990;168:406-413.
[Crossref] [Google Scholar] [PubMed]
- Everard M, Swarbrick A, Rigby A, Milner A. The effect of ribavirin to treat previously healthy infants admitted with acute bronchiolitis on acute and chronic respiratory morbidity. Resp Med. 2001;95:275-280.
[Crossref]
- Hung IFN, Lung KC, Tso EYK, Liu R, Chung TWH, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet. 2020;395:1695-1704.
[Crossref] [Google Scholar] [PubMed]
- Krilov LR. Safety issues related to the administration of ribavirin. Pediatr Infect Dis J. 2002;21:479-481.
[Crossref] [Google Scholar] [PubMed]
- Morrison G, Kotecha B, Evans J. Ribavirin treatment for juvenile respiratory papillomatosis. J Laryngol Otol. 1993;107:423-426.
[Crossref] [Google Scholar] [PubMed]
- Rigopoulou EI, Abbott WG, Williams R, Naoumov NV. Direct evidence for immunomodulatory properties of ribavirin on T-cell reactivity to hepatitis C virus. Antiviral Res. 2007;75:36-42.
[Crossref] [Google Scholar] [PubMed]
- Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2007.
- Wright PF. Progress in the prevention and treatment of RSV infection. N Engl J Med. 2014;776-777.
[Crossref] [Google Scholar] [PubMed]
- Smith RA, Kirkpatrick W. Ribavirin: A broad spectrum antiviral agent. 1980.
- Liu V, Dhillon G, Weill D. A multi‐drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart-lung transplant recipients. Transpl Infect Dis. 2010;12:38-44.
[Crossref] [Google Scholar] [PubMed]
- Phillips J. Inhaled efficacious dose translation from rodent to human: A retrospective analysis of clinical standards for respiratory diseases. Pharmacol Ther. 2017;178:141-147.
[Crossref] [Google Scholar] [PubMed]
- Muller RH, Mader K, Gohla S. Solid Lipid Nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161-177.
[Crossref] [Google Scholar] [PubMed]
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588-599.
[Crossref] [Google Scholar] [PubMed]
- Cheng YS, Moss OR. Inhalation exposure systems. Toxicol Meth. 1995;5:161-197.
- Zeitlinger M, Koch B, Bruggemann R, De Cock P, Felton T, Hites M et al. PK/PD of Anti-Infectives Study Group (EPASG) of the European Society of Clinical Microbiology, Infectious Diseases (ESCMID) Pharmacokinetics/pharmacodynamics of antiviral agents used to treat SARS-CoV-2 and their potential interaction with drugs and other supportive measures: A comprehensive review by the PK/PD of anti-infectives study group of the European society of antimicrobial agents. Clin Pharmacokinet. 2020;59:1195-1216.
[Crossref] [Google Scholar] [PubMed]
- Ishihara T, Kaneko K, Ishihara T, Mizushima T. Development of biodegradable nanoparticles for liver-specific ribavirin delivery. J Pharm Sci.2014;130:4005-4011.
[Crossref] [Google Scholar] [PubMed]
- Fasiolo LT, Manniello MD, Tratta E, Buttini F, Rossi A, Sonvico F. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur J Pharm Sci. 2018;113:2-17.
[Crossref] [Google Scholar] [PubMed]
- Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA. Fundamentals of aerosol therapy in critical care. Critical Care. 2016;20:1-16.
[Crossref] [Google Scholar] [PubMed]
- Walsh BK, Betit P, Fink JB, Pereira LM, Arnold J. Characterization of ribavirin aerosol with small particle aerosol generator and vibrating mesh micropump aerosol technologies. Respir Care. 2016;61:577-585.
[Crossref] [Google Scholar] [PubMed]
- Wyde PR, Wilson SZ, Petrella R, Gilbert BE. Efficacy of high dose-short duration ribavirin aerosol in the treatment of respiratory syncytial virus infected cotton rats and influenza B virus infected mice. Antiviral Res. 1987;7:211-220.
[Crossref] [Google Scholar] [PubMed]
- Shrimal P, Jadeja G, Patel S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem Eng Res Des. 2020;153:728-756.
- Tam JM, McConville JT, Williams RO, Johnston KP. Amorphous cyclosporin nanodispersions for enhanced pulmonary deposition and dissolution. J Pharm Sci. 2008;97:4915-4933.
[Crossref] [Google Scholar] [PubMed]
- Wong BA. Inhalation exposure systems: Design, methods and operation. Toxicol Pathol. 2007; 35:3-14.
[Crossref] [Google Scholar] [PubMed]
- Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92:740-746.
[Crossref] [Google Scholar] [PubMed]
- Han L, Wilson R, Slater S, Rutman A, Read R. Cole, In vitro and in vivo effects of ribavirin on human respiratory epithelium. Thorax. 1990;45:100-104.
[Crossref] [Google Scholar] [PubMed]
- Chemaly R, Aitken S, Wolfe C, Jain R, Boeckh M. Aerosolized ribavirin: The most expensive drug for pneumonia. Transpl Infect Dis. 2016;18:634-636.
[Crossref] [Google Scholar] [PubMed]
- Rauf A, Bhatnagar A, Sisodia SS, Khar RK, Ahmad FJ. Lungs deposition and pharmacokinetic study of submicron budesonide particles in Wistar rats intended for immediate effect in asthma. EXCLI J. 2017; 16:236-244.
[Crossref] [Google Scholar] [PubMed]
- Bernstein JM, Liss H, Erk SD. Comparison of oral and aerosol ribavirin regimens in the high risk elderly. J Clin Pharmacol. 1989;29:1128-1134.
[Crossref] [Google Scholar] [PubMed]
- Gilbert BE, McLeay MT. Mega Ribavirin aerosol for the treatment of influenza A virus infections in mice. Antiviral Res. 2008;78:223-229.
[Crossref] [Google Scholar] [PubMed]
- Adams RH, Christenson JC, Petersen FB, Beatty PG. Pre-emptive use of aerosolized ribavirin in the treatment of asymptomatic pediatric marrow transplant patients testing positive for RSV. Bone Marrow Transplant. 1999;24:661-664.
[Crossref] [Google Scholar] [PubMed]
- Hoffmann SH, Staffa JA, Smith RA, Craig DK, Parker G. Inhalation toxicity of ribavirin in suckling ferrets. J Appl Toxicol. 1987;7:343-351.
[Crossref] [Google Scholar] [PubMed]
- Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785-796.
[Crossref] [Google Scholar] [PubMed]
- Fernandez H, Banks G, Smith R. Ribavirin: A clinical overview. Eur J Epidemiol. 1986;2:1-14.
[Crossref] [Google Scholar] [PubMed]

